Hello Everyone,
This is my first post and I see that I have already mistakenly found out that by hitting tab you can accidentally post what you're typing too soon, and that apparently you cannot delete a post you just put up by mistake. I would like to reply to Autoholic's post regarding his question of the difference between using gas vs alcohol in his topic "Fuel Related Performance"
http://fepower.net/simplemachinesforum/index.php?topic=3477.0. I figured that I would make my response its own topic with its own title so that this might be easier to other users in the future to search for this information again.
In order to obtain my final credit hour during my senior year at University of Illinois in Mechanical Engineering, I did an independent study for one of my professors who taught the engineering of off-road vehicles. The topic of my study was the forced induction of internal combustion engines. In that paper I wrote about the chemistry of combustion and use of oxygenated fuels, amongst other topics. I figured that I would post the relevant pages here for others to read in case people found this useful.
I have to admit after re-reading it, that I should make the following qualifying statements:
1. The intended audience for this paper was my college professor, and as such it is somewhat heavy on the technical side, and some elements that are referenced in the paper are expected to have been known ahead of time and are not explained (for example what the 'lower heating value' of a fuel means). I will try to answer any questions regarding such things if anyone would like.
2. The analysis I conducted is based purely in the theoretical, mathematical, and scientific realm, and definitely not from a position of experience or measured data on a dyno for instance. I believe that Autoholic is more interested in that kind of data to support or contradict his own analysis (correct me if I am wrong here with that assumption).
With that here are the images of the pages (I have never imbedded images before so if it didn't work please let me know).
After reading this first page I figure I will add a few statements that may make the information more understandable or perhaps more relatable, that some readers may have questions on.
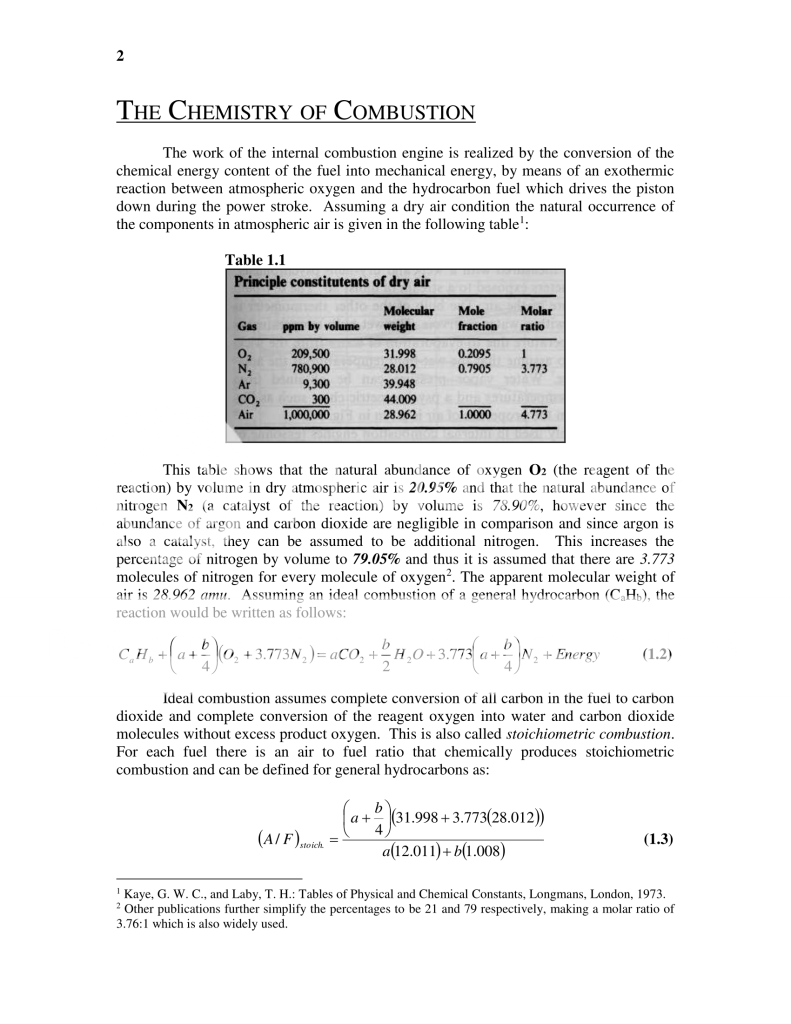
1. Gasoline (as well as other fuels like Diesel, kerosene, natural gas and propane) consists entirely of molecules that only contain hydrogen and carbon atoms, hence the term 'hydrocarbon fuel'. Since the amount of carbon and hydrogen atoms differs based on the fuel, a term is used to encompass all the fuels that fall into this category which is a 'general hydrocarbon' and is shown as a molecule with 'a' number of carbon atoms and 'b' number of hydrogen atoms:
CaHb2. For this analysis 'gasoline' that you would purchase at the pump is considered a 'practical fuel' which has too much variation for these equations to hold valid all the time. As such the practical fuel is reasonably approximated by a particular 'Pure Hydrocarbon' called
Iso-Octane which has the molecular structure of 8 carbon atoms and 18 hydrogen atoms:
C8H183. The stoichiometric (ideal combustion) equation
(1.2) for
Iso-Octane then becomes the following:
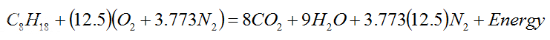
4. The stoichiometric air to fuel ratio of
Iso-Octane then becomes the following:
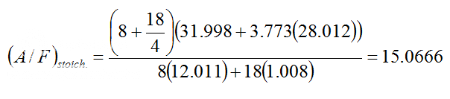
This shows that 15.0666 moles of air is required to stoichiometrically combust exactly 1 mole of Iso-Octane. This is slightly
leaner than that of actual Gasoline where other sources reference the stoichiometric A/F ratio as around 14.7 to 1.

The important information in page 2 here (numbered three in the actual document) is in the top and lower paragraphs. Understanding the technical definition of 'lean' vs 'rich' mixture is somewhat useful, but the really important information is in the concluding paragraph. Here I basically sum up that in a closed volume with a relatively fixed ratio of fuel to air (meaning that you can't stray too far from the calculated stoichiometric ratio and still achieve combustion), that the limiting factor on the amount of fuel that can be burned (and thus work and also power that can be generated) in that closed volume is the total amount of oxygen available. Once this is established a list is given of the three available ways to increase the amount of oxygen in the closed volume (ie. the cylinder).
The entire preceding two page analysis basically boils down to stating a very simple conclusion really, which is that you can't burn as much fuel as you want in the cylinder to increase power, and that the amount of fuel you can burn is limited entirely by how much oxygen you can get in there.Now moving onto the comparison to alcohol fuels...
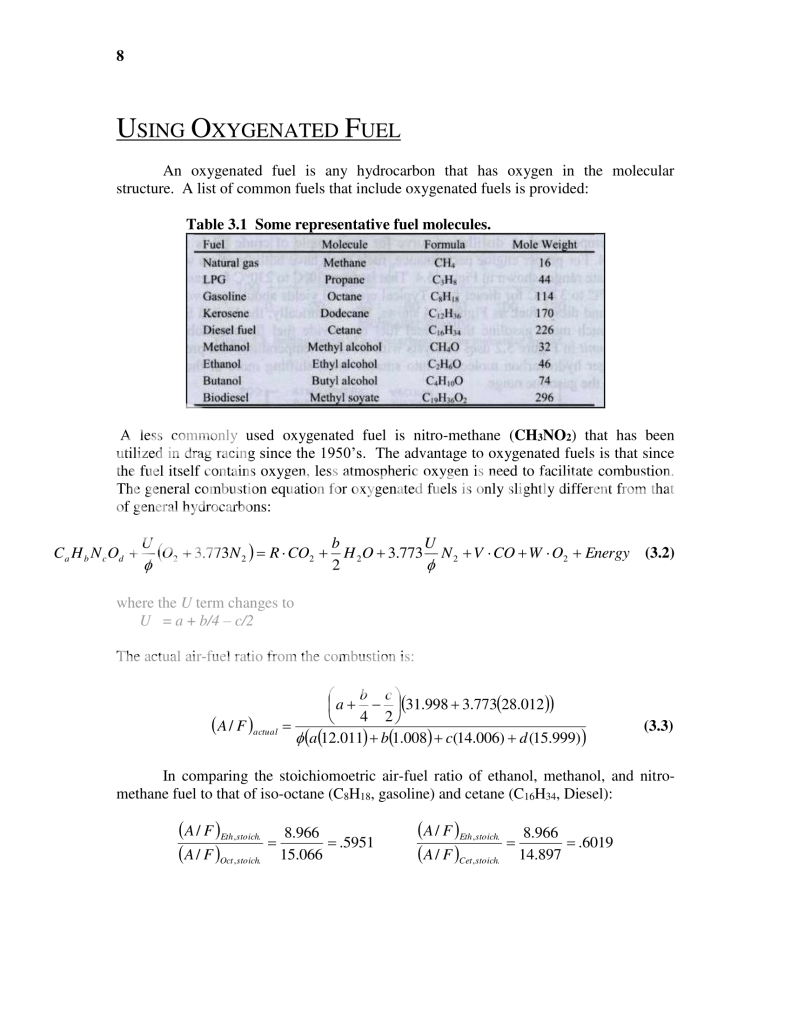
This first page is useful in that it defines what an alcoholic (oxygenated) fuel is, and how to go about calculating the stoichiometric air to fuel ratio depending on the chemical make up of that fuel. The importance of that information though is in the table on the next page...
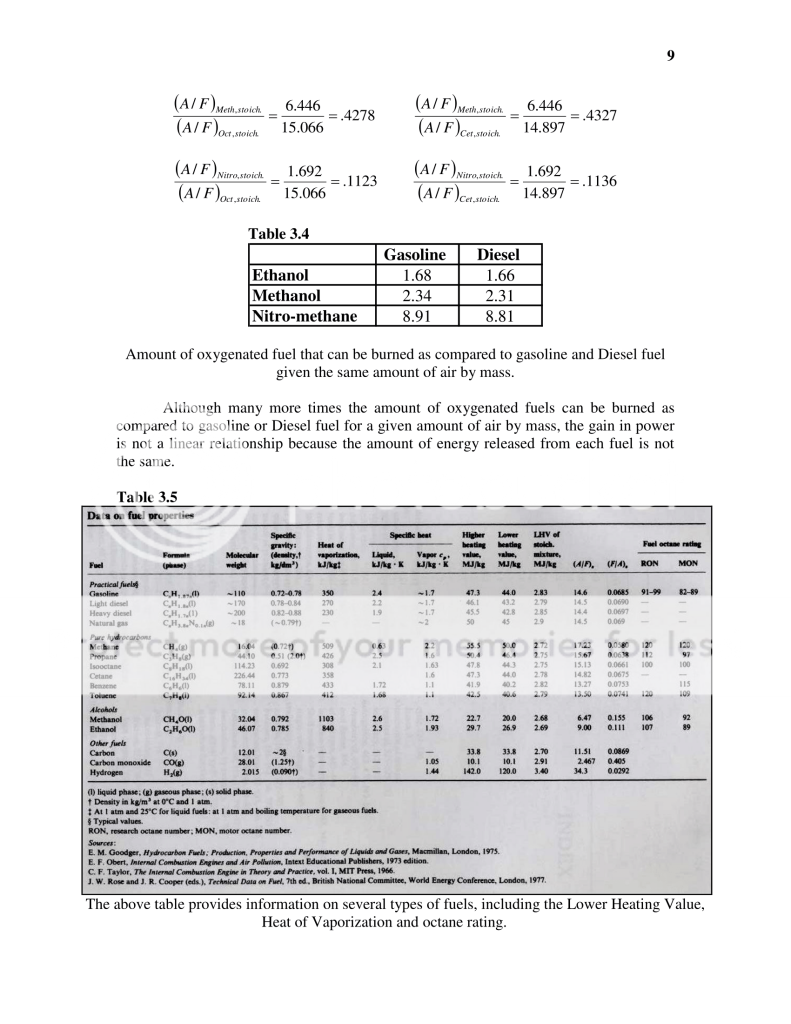
In table 3.4 I have simply taken the stoichiometric A/F ratio of
Iso-Octane (gasoline) and divided it by the stoichiometric A/F ratio of the listed alcoholic fuels. In comparing gas to nitro-methane for example I am showing that for the same mass of air being burned (lets say 1 slug **yes I just used the slug mass**....ok fine lets say 1 pound) that you can burn 8.91 times more nitro than gas. This DOES NOT mean however, that by switching to Nitro that you can expect to produce 8.91 times more horsepower.
Why?
Well if you look back at equation
3.2 you see that the in the combustion of these fuels that you not only get all these different molecular products like carbon dioxide or carbon monoxide etc. but that what makes the power is the
ENERGY released from the combustion, and the amount of energy released is not equal between different fuels being burned.
So the actual comparison of the power difference has to incorporate both the energy release as well as the A/F ratio of the fuels being compared...
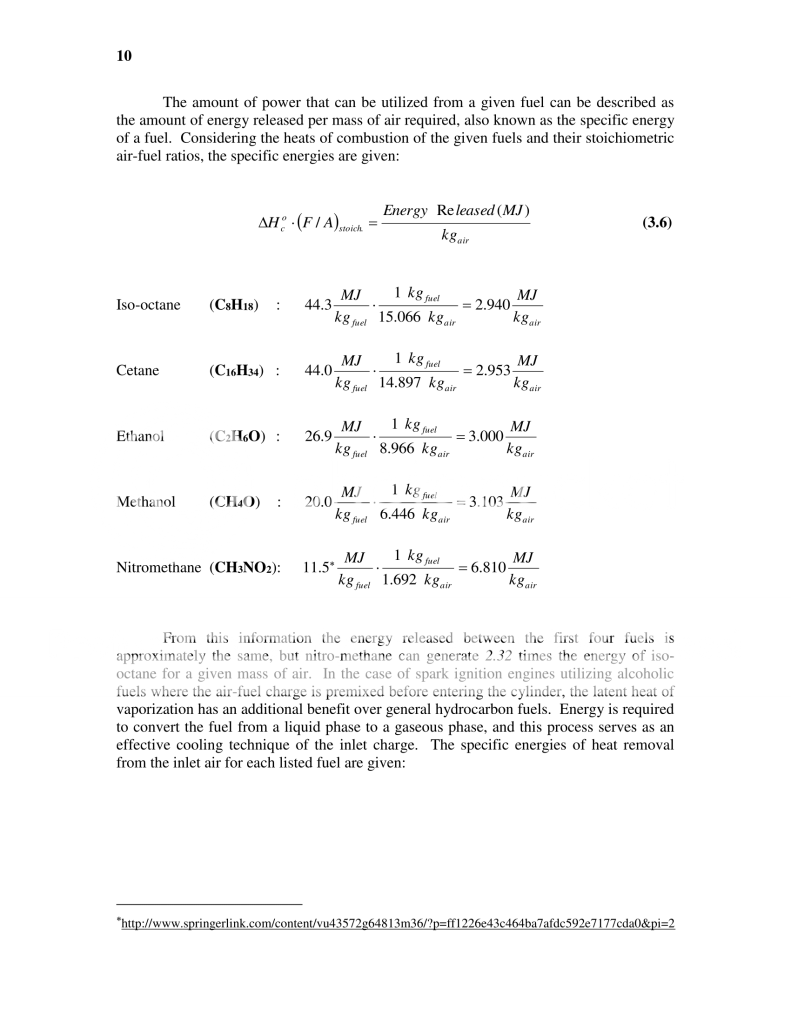
The conclusion seen here is that between gas, Diesel, Ethanol, and Methanol, that there is actually pretty little difference in how much energy can be released by the same mass of air, with the exception of Nitro. Again it isn't 8.91 times more powerful than gas, but actually 2.32 times more powerful.
In fact Jay stated in the topic created by Autoholic, that his experience shows that switching to methanol will net you approximately a 5% increase in power over gas (sorry but I haven't figured out how to quote yet), and based on the equations for the energy released by these different fuels the methanol would net a 5.5% increase in power compared to gas:
3.103/2.940 = 1.05544 = 5.544% increase.
This of course is only comparing if you were to take your existing engine and did not change any parameter to it (like compression ratio for instance) other than what fuel you burn in it and this is the approximate power gain you will see. The potential however is there to gain much more than that simple 5% from methanol for instance due to the fact that it can cool the inlet charge much more than gas can...
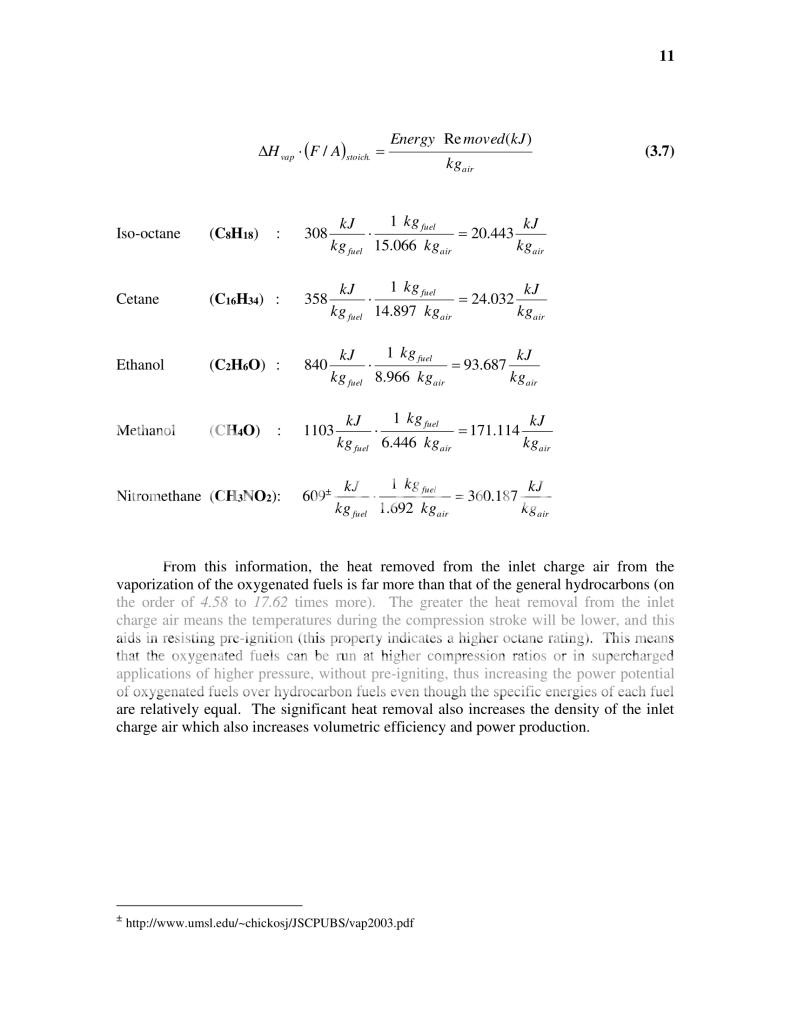
This means that by using an alcoholic fuel, I not only can get an immediate power increase from the energy release of the fuel itself, but I could also now allow myself to increase the compression ratio, or increase the boost pressure without pre-igniting the fuel.
I hope that my edits over the course of the day has helped some readers understand some of this a little better.
Thank you for welcoming me into your forum,
Calvin